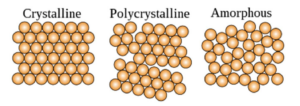
This causes many gemstones to have naturally flat sides that are highly uniform even at the macro and microscopic scale. Most inorganic compounds however are polycrystals, meaning they are composed of many microscopic crystal structures which gives them some molecular uniformity [3]. Several examples of polycrystals include most metal, rocks, ceramics, and ice. These are very different from amorphous solids, which do not have any organized structure, and tend to be much weaker in comparison. Some examples of amorphous solids are glass, wax, and many plastics [3]. Figure 1: This shows the distinct differences between the molecular structures of crystalline, polycrystalline, and amorphous substances. Image located at https://en.wikipedia.org/wiki/Crystal Concrete Hydrates: The DNA of Cement Concrete has a polycrystalline structure and is composed of three molecules: silica tetrahedrons (silicon-oxygen tetrahedrons), calcium oxide, and water [4].
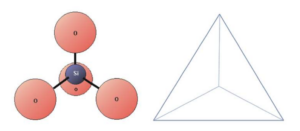
Figure 2: A silica tetrahedral pictured next to its geometric representation, the tetrahedron. Image located at https://openeducationalberta.ca/practicalgeology/chapter/3-1-silicate-mineral-groups/
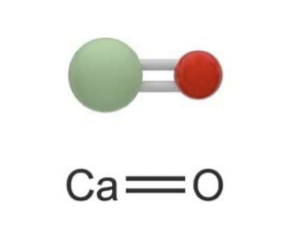
Figure 3: A calcium oxide molecule. Image adapted from https://www.shutterstock.com/search/calcium-oxide

Figure 4: A water molecule. Image adapted from https://www.dreamstime.com/illustration/molecule.html Please note that the tetrahedron representation in figure 2 (triangular pyramid) DOES NOT represent what the silicon-oxygen molecule actually looks like. Imagining each as a tetrahedral allows us to think of the geometry of many of these molecules joined together in a much easier manner as opposed to showing the Lewis dot structure that makes these visual representations more confusing than helpful. With that in mind, observe how these tetrahedral structures might look when they are bonded.

Figure 5: Top-down view of a geometric representation of one layer of silica molecules in a crystal. Image located at https://ecampusontario.pressbooks.pub/geology/chapter/2-4-silicate-minerals/ This image shows a simplified birds-eye view of a single sheet of bonded silica tetrahedrons. When studies were conducted on concrete however, researchers found that its structure is not so regular. Every 2nd, 5th, or 8th tetrahedron points in the direction opposite to the other tetrahedrons [4]. An example of this behavior is shown with slightly different materials that also have somewhat irregular crystal structures in figure 6.
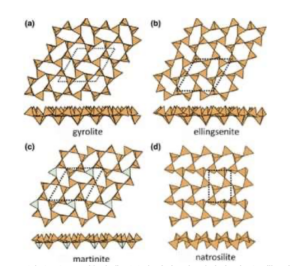
Figure 6: Various crystal structures with similar tetrahedral molecule behavior to silica. Image located at https://www.researchgate.net/figure/Planar-6-3-sheets-of-u-d-tetrahedra-in-a-gyrolite-b-ellingsenite-c-martiniteand_fig8_339616565 That critical piece of information can explain why concrete has a polycrystalline structure rather than a crystalline structure, but now let’s look specifically at concrete powder:
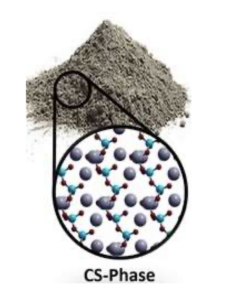
Figure 7: Concrete powder adjacent to its molecular representation. Image adapted from https://chemistryeurope.onlinelibrary.wiley.com/doi/full/10.1002/chem.201705974 The silicon atoms (blue) form 4 bonds to surrounding oxygen atoms (red) that are very kindly being shared by the calcium atoms (gray) which form 2 bonds [1]. Every atom “wants” to form an appropriate number of bonds in order to reach the lowest state of energy that is possible. This sometimes causes unwanted bonds to occur such as two silicon atoms sharing one oxygen atom. As a result, notice that the concrete powder alone has no thoroughly organized structure. There is however a solution to this issue… just add water [1].
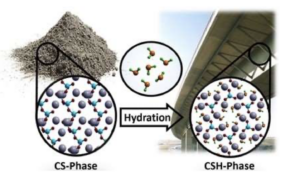
Figure 8: A molecular representation of concrete before and after the hydration reaction occurs. Image located at https://chemistry-europe.onlinelibrary.wiley.com/doi/full/10.1002/chem.201705974 Now that water is present, every calcium atom (ideally) attaches a water molecule to itself while also sharing its oxygen to a silicon atom. After this reaction occurs, you can begin to see this tetrahedron sheet start to form. Notice the placement of each silicon atom, there are six of them spread out in a hexagon-like pattern which is very similar to the pattern in figure 5. Concrete Doesn’t Dry, it Crystalizes Understanding all the prerequisite information above will now allow us to identify why concrete has a curing process. After water has been added to cement powder, the hydration reaction begins to occur and will proceed over an extended period [2]. The water molecules attach themselves to the calcium atoms thus causing these microcrystals to form from groups of the calcium-silicate-hydrates [4]. The concrete will have a solid composition 24-48 hours after the reaction initially began which conveniently allows humans to walk across its surface, but many changes are still occurring at the atomic level [2]. The microcrystals are still being formed by using available water molecules to hydrate the calcium atoms. This is why you must supply water to the surface of fresh concrete. This reaction also requires energy as does any process, but this can be supplied as heat from the surroundings which is why concrete must cure at an appropriate temperature (between 50℉ and 85℉). The reaction slows down significantly at 50℉ and stops almost completely at 40℉ [2]. The adequate time for the hydration to be fully complete is 28 days which can ensure that the material has had enough time to form as many microcrystals as possible. Properly cured concrete that has thoroughly developed it’s polycrystalline structure will not as easily crack and will be resistant to abrasion [2]. Why Can’t I Paint New Concrete? By painting over new concrete, you are effectively ruining the chance that your pavement will last for as long as you intend it to. Putting paint down and sealing it will greatly inhibit the ability of water to soak into the concrete which is the only method by which the hydration reaction can proceed. Patience in this case is a virtue and will be greatly rewarded. You’ll save a significant amount of money in the long run if you can manage to wait for the duration of the curing process to end before considering putting any paint on your concrete.
References [1] P. Thissen, C. Natzeck, N. Giraudo, P. Weidler, and C. Woll, “Hydration of Concrete: The First Steps,” Chemistry Europe -European Chemical Societies Publishing, 12-Apr2018. [Online]. Available: https://chemistry-europe.onlinelibrary.wiley.com/. [Accessed: 16-Nov-2022]. [2] B. Palmer, “Concrete curing time: How long does concrete take to dry?,” The Concrete Network, 19-Aug-2022. [Online]. Available: https://www.concretenetwork.com/curingconcrete/. [Accessed: 16-Nov-2022]. [3] “Crystal,” Wikipedia, 28-Oct-2022. [Online]. Available: https://en.wikipedia.org/wiki/Crystal. [Accessed: 16-Nov-2022]. [4] D. Brehm, “Cement’s basic molecular structure finally decoded,” MIT News | Massachusetts Institute of Technology, 09-Sep-2009. [Online]. Available: https://news.mit.edu/2009/cement-0909. [Accessed: 16-Nov-2022]. [5] S. Earle, “2.4 silicate minerals,” Physical Geology, 01-Sep-2015. [Online]. Available: https://opentextbc.ca/geology/chapter/2-4-silicate-minerals/. [Accessed: 16-Nov-2022]. [6] S. McGoldrick, “3.1 silicate mineral groups,” A Practical Guide to Introductory Geology, 10-May-2021. [Online]. Available: https://openeducationalberta.ca/practicalgeology/chapter/3-1-silicate-mineral-groups/. [Accessed: 16-Nov-2022].
